Flow chemistry is a term used to describe a chemical reaction run in a continuous flow stream.
As a methodology, continuous flow offers potential for the efficient synthesis of chemical products and can offer a number of advantages over traditional batch methods.
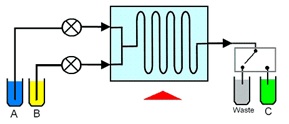
To carry out a reaction in flow, reactants are first pumped into a mixing device. The flow continues through a reactor, where the physical conditions are set to initiate the chemical reaction until the reagent flows out of the reactor, and the reagents/products move into an environment that does not promote reaction. The reactor can be a simple pipe, tube or complex micro-structured device, and the reactants may also be exposed to an electrical flux or a photon flux to promote an electrochemical or photochemical reaction.
In Flow chemistry, reagents are pumped under pressure and flow continuously through the reactor, which contrasts with batch reactors where all reagents are loaded into a vessel at the start. Reaction time is determined by the time the reagents take to flow through the reactor. When the products flow out of the reactor they move away from the environment where reaction can occur, helping to prevent unwanted reactions from taking place.
This time spent inside the reactor, and therefore exposed to the reactive environment, is called the residence time. Reaction stoichiometry is controlled by the relative flow rates of the reactants; the concentration of one reagent relative to another can be increased simply by pumping that reagent at a higher flow rate. Flow reactors have excellent heat transfer when compared with batch reactors, due to the much greater surface area to volume ratio of flow reactors over batch reactors. Reactors designed for flow chemistry have high rates of mass transfer, because of the small sizes and good mixing that is possible.
Flow chemistry offers the chemist precise control of the four critical reaction parameters: Stoichiometry, mixing, temperature and reaction time. When reactions are run in continuous flow only small quantities of potentially hazardous materials are exposed to each other, and the reaction environment, so processes can be made safer. Reactive intermediates don’t need to be isolated, flow reactions can be easily run in sequence or “telescoped”, removing the need for what are often complicated and laborious stages of a batch synthesis.
Flow chemistry is particularly advantageous for reaction control and scale-up of photochemical and electrochemical reactions. Photochemical reactions benefit from significantly more efficient light penetration and electrochemical reactors have minimal anode/cathode separation, reducing the need for electrolyte and enabling rapid mass-transfer.