Electrochemistry at Vapourtec has come a long way in just a few short months! Our electrochemical reactor, now known as the Ion, can now operate under a wide range of temperatures, pressures, and using a variety of different electrode materials.
I’ve admitted before that my experience with electrochemistry is limited, and looking through the literature I feel I would be forgiven if I thought that electrode choice didn’t make a difference; especially when using materials such as carbon – I don’t mean electrode pairs, which of course will make a difference as different metals/conductors have different surface potentials.
Throughout the literature, graphite is often called upon to act as an electrode and, more recently, carbon-loaded polymers such as PVDF. The Ion has been designed to take a wide range of electrodes, and as carbon is available in so many different incarnations I thought it would be interesting to trial a bunch of different carbon electrodes under the same electrochemical conditions to see if it makes any difference.
It does.
Take a look below; the chemistry is a very commonly studied model reaction, oxidising 4-tertbutyl toluene with methanol to the methyl ether, then the acetal (the aldehyde can crop up as well (see the peak at around 6.4 mins?), but after extensive investigation I found that the aldehyde chromophore is 10 x more strongly absorbing than the toluene, so it’s never quite as bad as it looks).
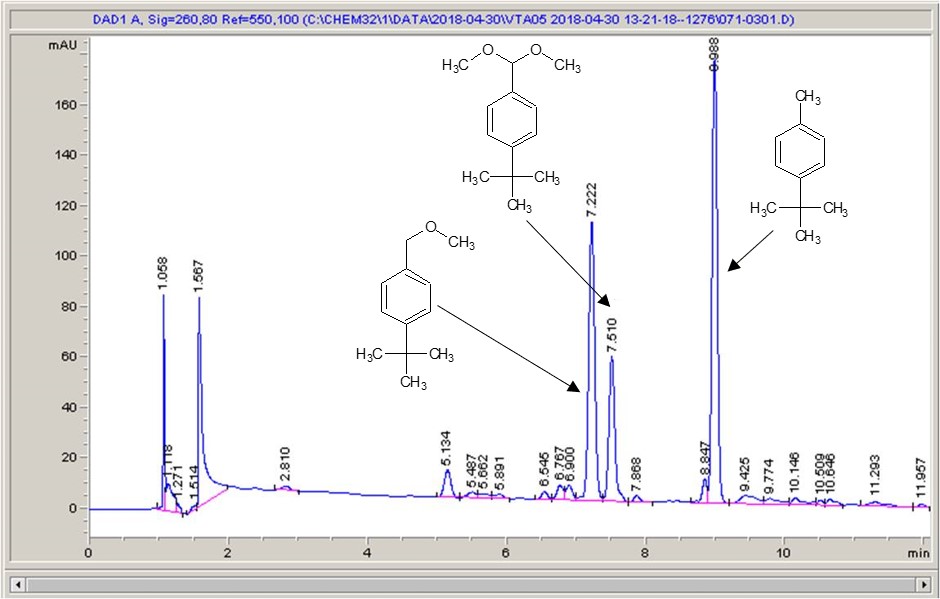
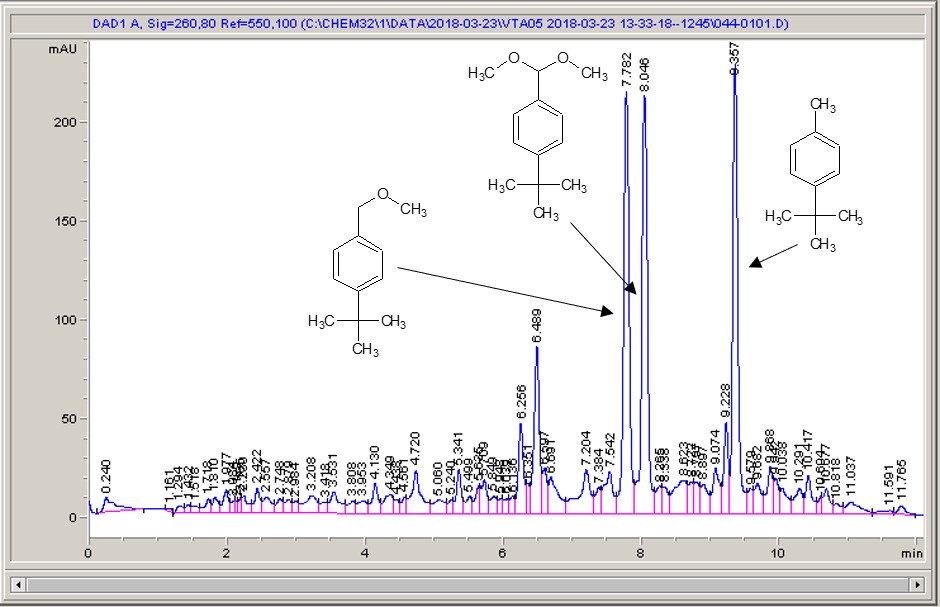
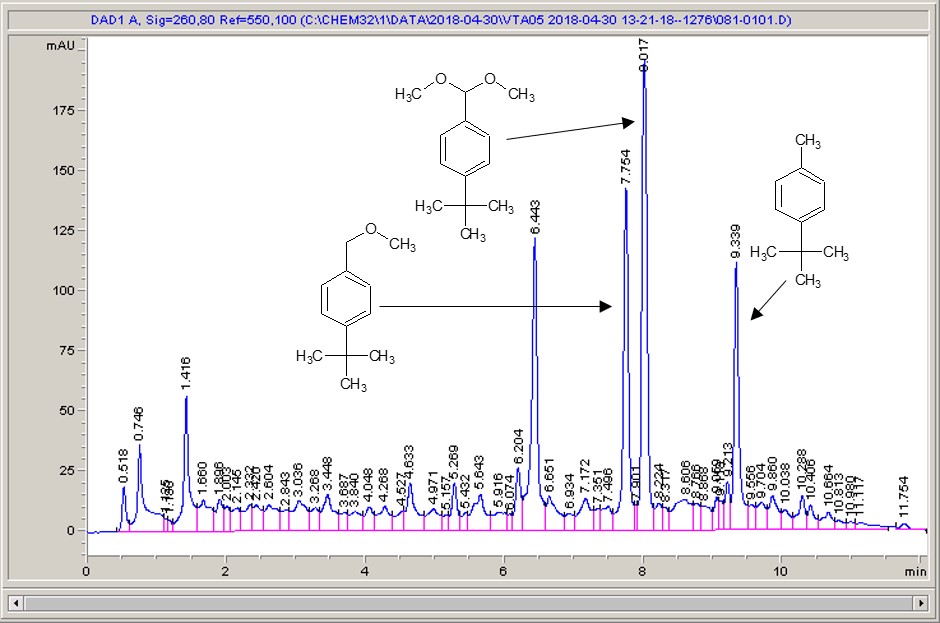
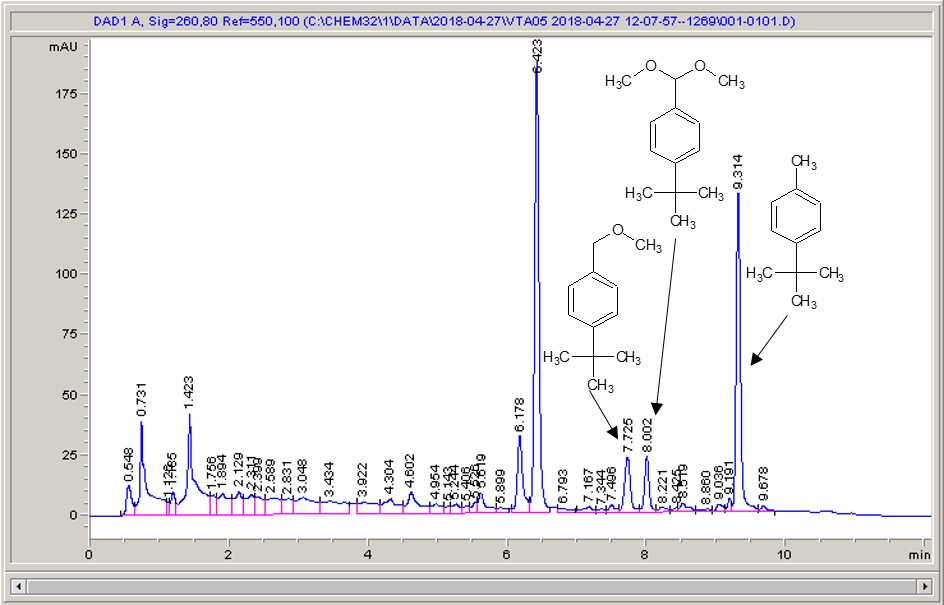
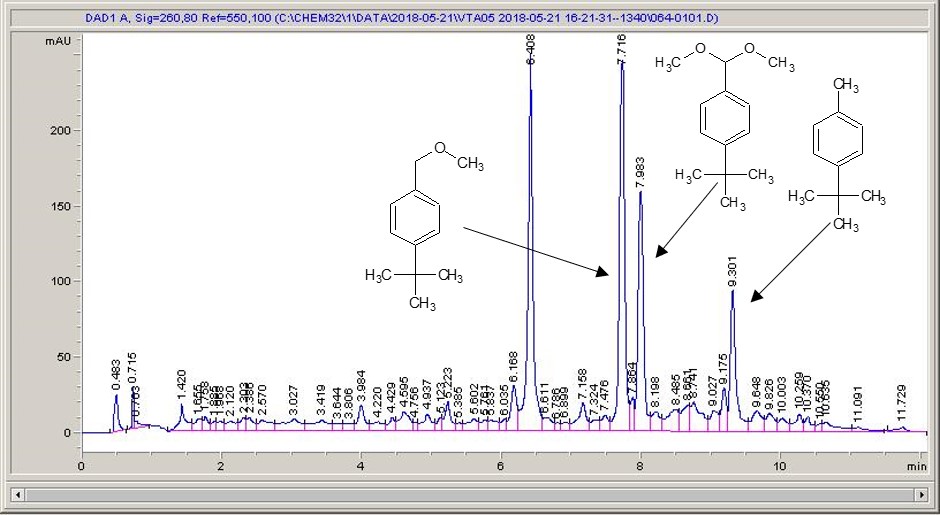
What I see right away is a big difference between each of the different materials. Carbon-loaded PVDF (material number 4) has resulted in a rather poor conversion with a significant amount of the aldehyde (even accounting for the stronger chromophore), whereas some of the other materials have quite high conversions, and some show the start of interesting selectivities. The baseline noise changes considerably between electrodes as well. Each measurement is preceded by a blank solvent run for comparison, and they always come through clean.
It just goes to show – not all carbon is equal…
