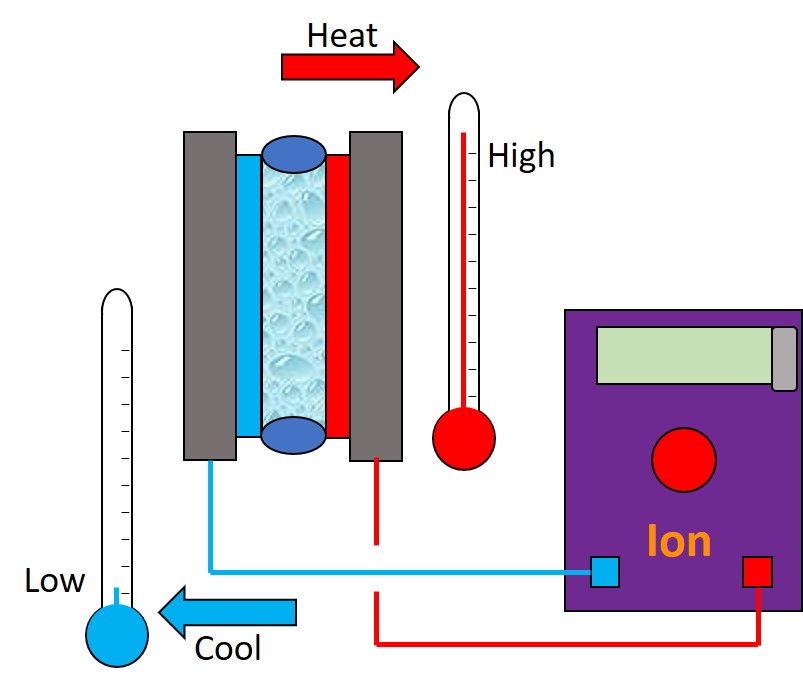
Recently, much of my work has been focussed on using the Vapourtec Ion electrochemical reactor; one of the key features I’ve been investigating is control of temperature. The Ion can be heated well above ambient and cooled to below zero (as we’re still before the official release, I can’t tell you the absolute values), which opens up a vast chemical reaction space to explore. A quick search of the literature, which is already pretty light on electrochemical syntheses, shows that the vast majority of electrochemical reactions are carried out at room temperature; and there are very few examples of cooling an electrochemical reaction.
I suppose the most immediate question is “does temperature have any effect on an electrochemical reaction?” to which I can direct you to a couple of very interesting publications: A group at Pfizer published in 2017 that by incorporating electrochemistry into a nickel-catalysed cross-coupling, they were able to optimise the reaction with much greater control and achieve better conversions and selectivities than with the analogous activated metal powder reactions. The key point here is that the reaction is a thermally mediated coupling, taking place at 65 degrees, but the electrochemistry is used to help control and direct the outcome. A second paper earlier this year from Keio University, Japan, describes a palladium-catalysed iodination performed at 90 degrees. Addition of electrochemical oxidation improved the rate of iodination at hindered positions, and enabled the researchers to direct the reaction to one product over another. Exciting stuff, and both need the ability to heat the electrochemical reactor.
I think there are a number of other phenomena that are temperature dependent to consider: molecules adsorbing to electrode surfaces, followed by ions desorbing; changes in viscosity affecting rates of ion diffusion; the potential for non-electrochemical side reactions – all are worth considering and most probably influenced by the reaction temperature. Certainly in light of the recent literature, combining electrochemistry with thermal processes offers fascinating potential for directing the outcome of reactions.
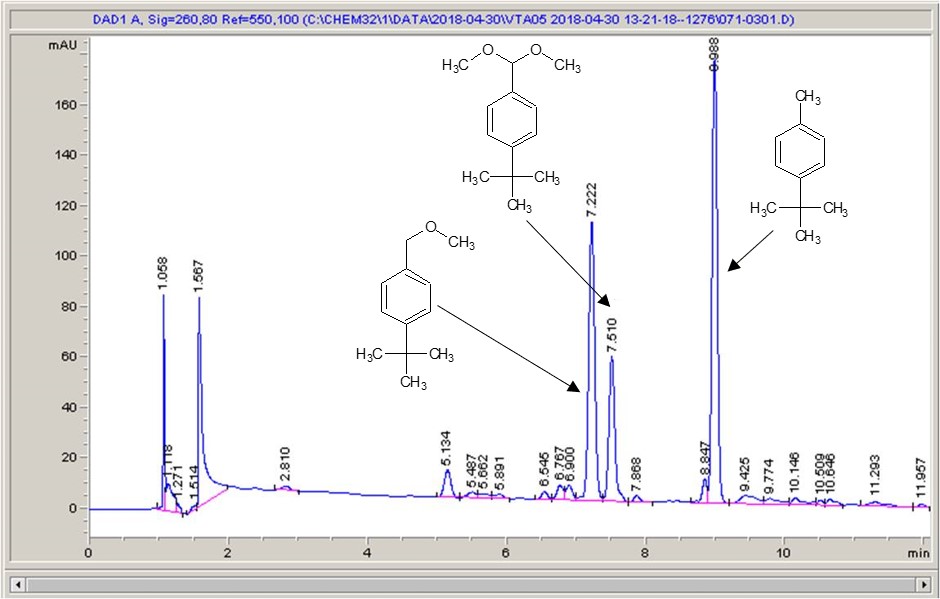
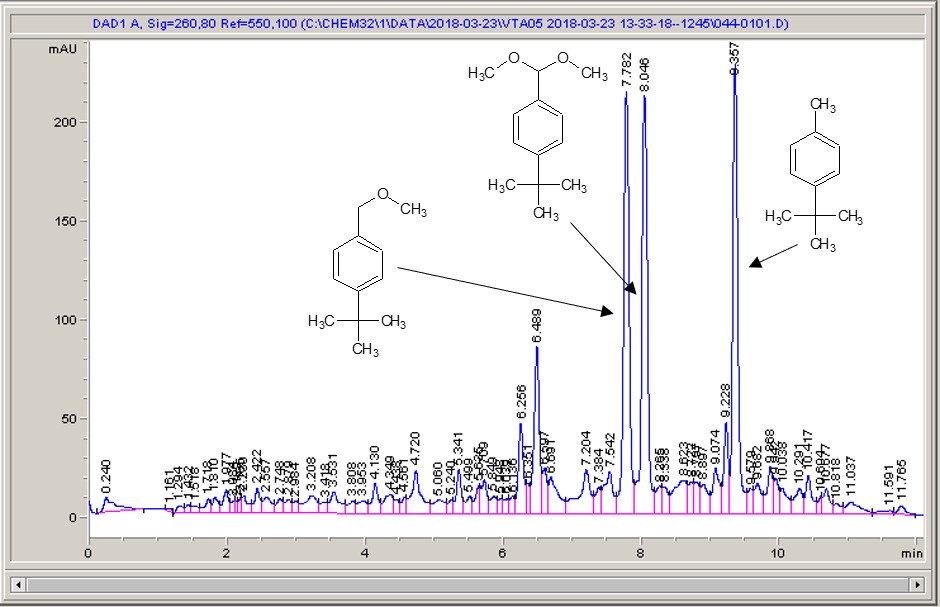
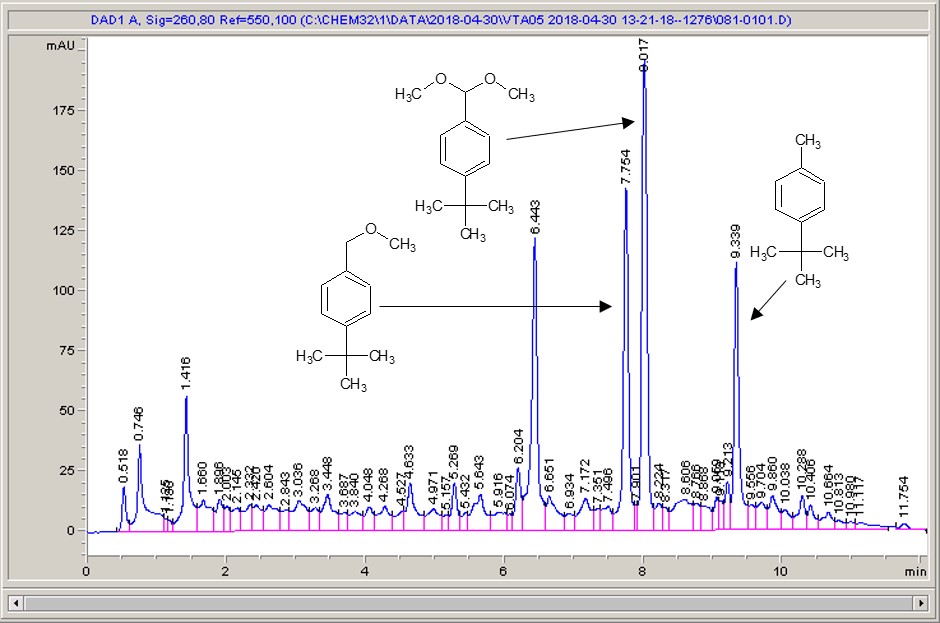
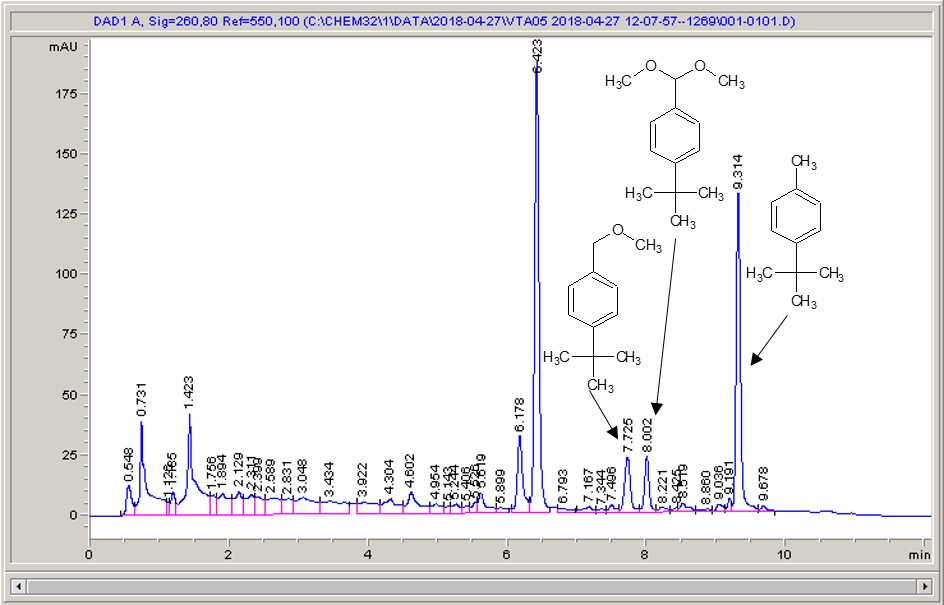
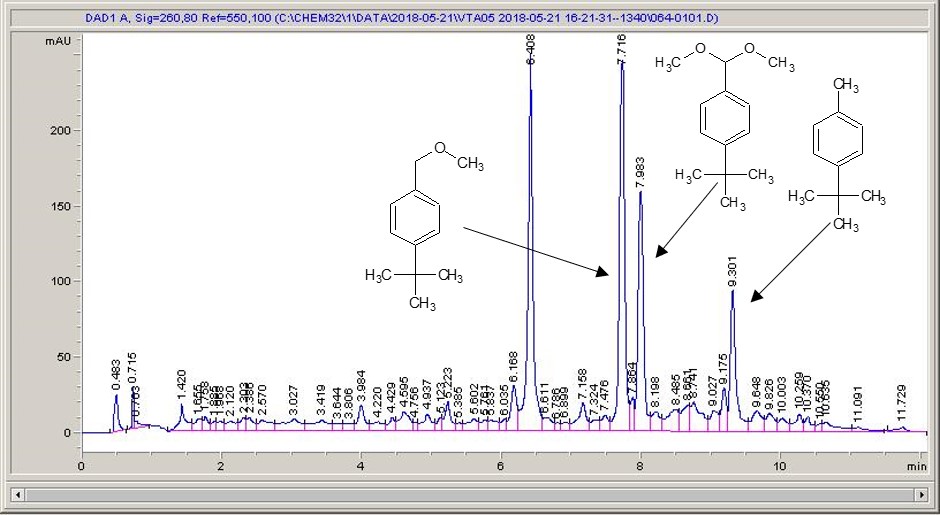
Below are some HPLC traces for my model reaction – the methylation of 4-t-butyl toluene that I’ve written about before. The key point with these experiments is that methanol (reagent and solvent) is taken well beyond its boiling point in the high temperature example. This is only possible because the Ion has been designed to operate at pressure, specifically to allow super-heating of the solvent.
Looking at the results below (I’ve discussed elsewhere that the largest peak, due to an aldehyde, has a much stronger chromophore than the other materials – I have adjusted for this in my integration which is why the apparent peak areas don’t quite much the percentages), the influence of temperature is marginal for this reaction under these conditions.
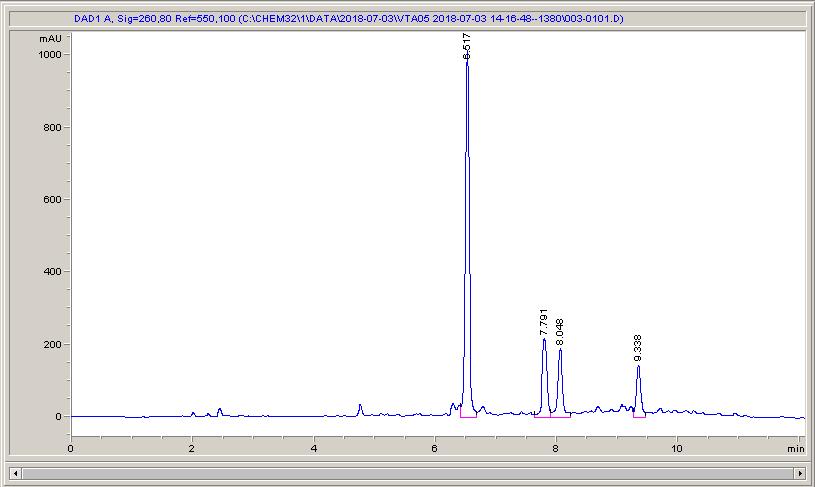
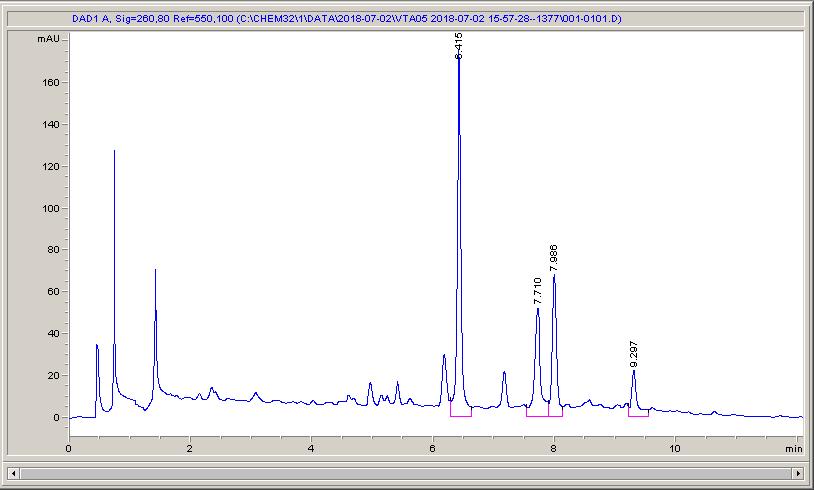
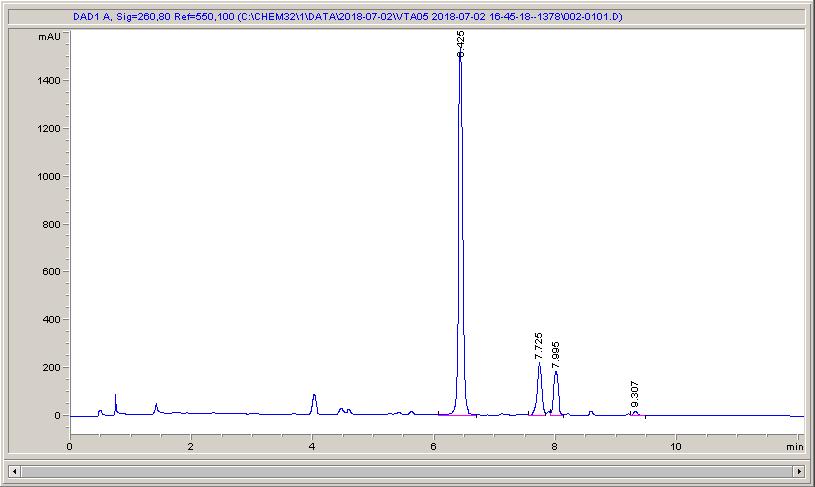
What I think is most exciting is the limited information in the literature regarding the effect of temperature on these reactions.
Pass me my pith helmet – there’s some exploring to do!
For more information about Vapourtec, click here.
Return on the 12th of September for the next blog about measuring the electrical behaviour inside the Ion